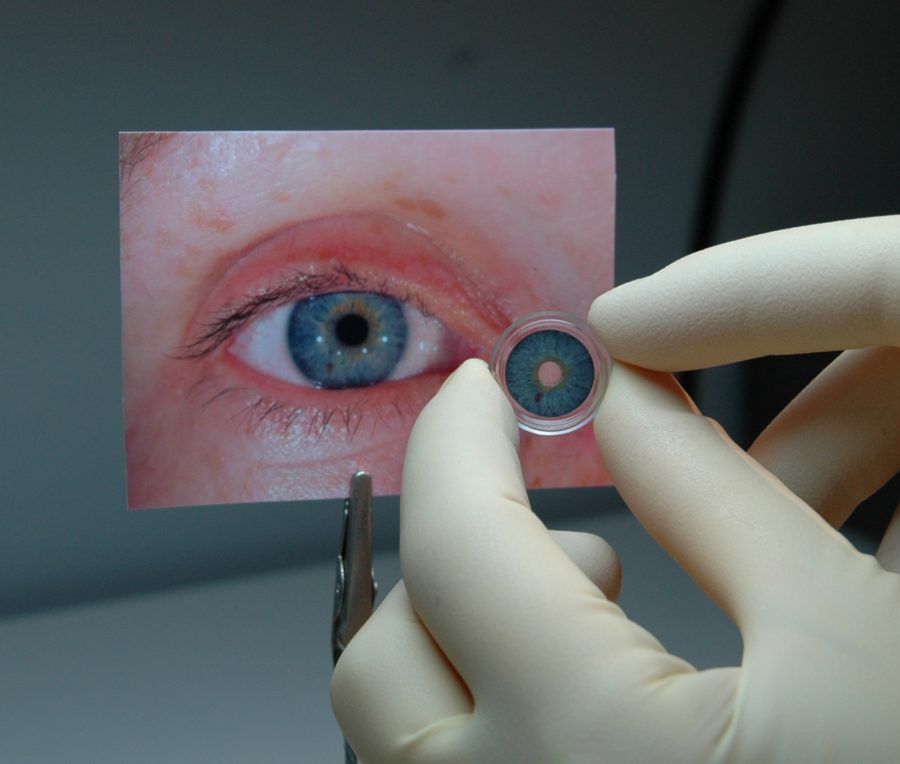
The first stand-alone prosthetic iris has received regulatory approval in the United States. CustomFlex Artificial Iris (HumanOptics) is a flexible silicone device indicated for adults and children with congenital aniridia or iris defects due to albinism, traumatic injury or surgical removal.
“Today’s approval of the first artificial iris provides a novel method to treat iris defects that reduces sensitivity to bright light and glare,” said Malvina Eydelman, MD, director of the division of ophthalmic and ear, nose and throat devices at the FDA’s Center for Devices and Radiological Health. “It also improves the cosmetic appearance of the eye in patients with aniridia.”
According to HumanOptics, the custom prosthesis is handcrafted so that its color matches the fellow eye. The pupil size is fixed at 3.35 mm and features an undulated edge that replicates the pupillary frill of a natural iris.
The implant is cut to size using a trephine and inserted into the ciliary sulcus using a sclerocorneal approach, as seen in this clinical video by Samuel Masket, MD. The prosthesis can also be placed through the open sky during penetrating keratoplasty.
The FDA’s decision was based on results from a non-randomized clinical trial of 389 adult and pediatric patients with aniridia or other iris defects. After surgery, more than 70% of patients reported a significant decrease in light sensitivity and glare. Investigators also found significant improvements in health-related quality of life and high satisfaction rates (94%).
The findings were similar to those from a 32-patient retrospective case-series published in the May 2016 issue of Ophthalmology. Both studies reported a low rate of adverse events associated with the device and surgical procedure. Complications included dislocation, strands of device fiber in the eye, increased IOP and iritis.
Original Source: American Academy of Ophthalmology
